- Veterinary View Box
- Posts
- New Imaging Insights: CT & Ultrasound Patterns That Distinguish FGESF in Cats
New Imaging Insights: CT & Ultrasound Patterns That Distinguish FGESF in Cats
VRU 2025
Hongji Yoon, Myounghun Kim, Hayoung Lim, Seungjun Lee, Minsu Lee, Joohyun Jung, Jungha Lee, Changgyu Im, Sanghee Lee, Jaehwan Kim, Kidong Eom
Background
Feline gastrointestinal eosinophilic sclerosing fibroplasia (FGESF) is a non-neoplastic, mass-forming condition characterized by dense collagen deposition and eosinophilic inflammation. It is increasingly recognized in young to middle-aged cats, yet imaging descriptions remain limited. While ultrasonography has been reported in prior small studies, CT features have only been described in case reports. Given FGESF’s clinical mimicry of neoplasia and its variable anatomical presentation, the authors aimed to comprehensively characterize CT and ultrasonographic findings in affected cats and assess concurrent abnormalities to improve diagnostic accuracy.
Methods
This retrospective multicenter study analyzed records from 10 veterinary hospitals (2020–2024). Sixteen cats with histopathologically confirmed FGESF that had both pre- and postcontrast CT and ultrasonography were included. Lesion location, morphology, layering, contrast enhancement, echogenicity, and quantitative CT parameters were assessed. Concurrent abnormalities—including lymphadenopathy, obstruction, perforation, and peritoneal changes—were evaluated. Statistical analyses focused on relationships between anatomical location and growth patterns, with significance set at p < 0.05.
Results
FGESF primarily affected young to middle-aged cats (median 3.5 years). Most lesions were gastrointestinal (14/16), frequently involving the proximal duodenum, jejunum, and pyloric regions. Growth pattern correlated significantly with location: proximal duodenum and pylorus consistently showed endophytic expansion. CT commonly revealed heterogeneous contrast enhancement (86%), mucosal layer enhancement (86%), and ulceration (50%). Ultrasonography consistently demonstrated intraparenchymal hyperechoic foci (100%), heterogeneous echotexture (93%), and mixed echogenicity (93%), with loss or alteration of wall layering in all cases. Marked lymphadenopathy occurred in 85% of cats, occasionally showing cavitation. Gastrointestinal perforation was identified in two cats, both of which died within 24 hours postoperatively.
Limitations
Limitations included the small sample size inherent to this rare disease, heterogeneity in CT protocols across institutions, and incomplete long-term follow-up and treatment documentation. Additionally, lack of comparative CT datasets for feline neoplasia restricts the ability to directly contrast FGESF with other mass-forming diseases.
Conclusions
CT and ultrasonography reveal characteristic yet variable imaging features of FGESF. Endophytic growth in the proximal duodenum and pylorus may aid differential diagnosis. Frequent findings include heterogeneous enhancement, mucosal enhancement, ulceration, mixed echogenicity, and hyperechoic intralesional foci. Marked lymphadenopathy is common, and cavitary nodes may reflect FGESF infiltration. Perforation, while rare, portends grave prognosis. The study supports including FGESF among differentials for feline gastrointestinal masses and highlights the diagnostic utility of combining CT and ultrasonography, while emphasizing the ongoing need for histopathological confirmation.
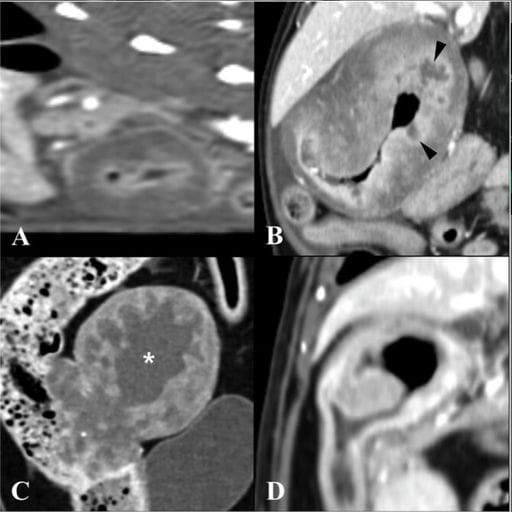
Postcontrast sagittal (A) and dorsal (B–D) CT images demonstrating different lesion shapes in FGESF. (A) Circumferential symmetric lesion at the pyloroduodenal junction (PDJ) showing homogeneous contrast enhancement with mucosal layer enhancement. (B) Circumferential asymmetric endophytic growth in the proximal duodenum showing heterogeneous contrast enhancement. Ulcerated regions are indicated (black arrowheads). (C) Noncircumferential exophytic growth in the descending colon, showing heterogeneous enhancement with a cavitated region (white asterisk). (D) Noncircumferential endophytic growth in the proximal duodenum. CT, computed tomography; FGESF, feline gastrointestinal eosinophilic sclerosing fibroplasia.
How did we do? |
Disclaimer: The summary generated in this email was created by an AI large language model. Therefore errors may occur. Reading the article is the best way to understand the scholarly work. The figure presented here remains the property of the publisher or author and subject to the applicable copyright agreement. It is reproduced here as an educational work. If you have any questions or concerns about the work presented here, reply to this email.