- Veterinary View Box
- Posts
- Novel VDR Gene Mutation Identified in Kitten With Vitamin D–Resistant Rickets
Novel VDR Gene Mutation Identified in Kitten With Vitamin D–Resistant Rickets
Journal of Veterinary Medical Science 2025
Sora Suzuki, Hitomi Masuyama, Jiro Miyamae, Hiroaki Hemmi, Naoaki Matsuki, Kohei Murakami
Background
Vitamin D-dependent rickets results from inadequate mineralization of developing bone due to impaired vitamin D metabolism or signaling. While cats rely exclusively on dietary vitamin D, hereditary defects in vitamin D–activating enzymes or in the vitamin D receptor (VDR) can cause vitamin D-dependent rickets types I and II. Only ten feline cases have been previously reported, and few have documented underlying genetic mutations. Dysfunction of VDR leads to VDDR-II, characterized by elevated 1,25(OH)₂D₃ despite hypocalcemia and secondary hyperparathyroidism. This study describes a feline case suspected of VDDR-II and provides genetic and functional evidence of a novel missense VDR variant.
Methods
A clinical evaluation, including radiography, serum biochemical analysis, ionized calcium, intact parathyroid hormone (iPTH), vitamin D metabolite quantification, and urinalysis, was performed. Genomic DNA sequencing of all 11 VDR exons was conducted using PCR amplification and Sanger sequencing. In silico pathogenicity prediction tools were applied to identified variants. Functional testing was performed by generating VDR-deficient feline kidney epithelial cells (CRFK-VDR⁻) via CRISPR-Cas9, then rescuing these cells with vectors expressing either wild-type or mutant VDR. Responsiveness to 1,25(OH)₂D₃ was assessed by CYP24A1 mRNA induction.
Results
An inbred 10-month-old kitten presented with lameness, inability to close the mouth, marked skeletal pain, and radiographic signs of generalized osteopenia, widened growth plates, and temporomandibular joint dysplasia. Laboratory tests revealed severe hypocalcemia with marked elevation of iPTH and strikingly increased 1,25(OH)₂D₃ levels, indicating vitamin D resistance. Despite aggressive calcium and vitamin D analog supplementation, calcium remained low, further supporting VDDR-II. Sequencing identified three VDR variants—c.439A>G, c.509C>T, and c.529_530insGCA—altering amino acids Thr147Ala, Pro170Leu, and Gly177_Gly178insSer. In silico tools predicted Pro170Leu to be damaging. Functional assays demonstrated that mutant p.Pro170Leu VDR failed to induce CYP24A1 in response to physiological concentrations of 1,25(OH)₂D₃, whereas wild-type VDR restored responsiveness. Higher doses of 1,25(OH)₂D₃ produced only weak activation in mutant-expressing cells. The cat was diagnosed with VDDR-II and, with long-term calcium supplementation, exhibited clinical improvement though some skeletal limitations persisted.
Limitations
One identified variant (p.Gly177_Gly178insSer) was not functionally evaluated, leaving its contribution unresolved. Absence of feline-specific VDR antibodies prevented protein-level confirmation of VDR deficiency in CRFK-VDR⁻ cells. As a single case, generalizability is limited. Initiation of therapy at nearly one year of age may have influenced long-term skeletal outcomes, complicating interpretation of treatment response severity relative to earlier-reported cases.
Conclusions
The authors identify a novel missense mutation (p.Pro170Leu) in the feline VDR gene that results in impaired receptor function and causes VDDR-II. Functional evidence confirmed that the mutant receptor responds poorly to 1,25(OH)₂D₃, establishing the pathogenicity of the variant. This case demonstrates the diagnostic value of combining genetic testing with functional receptor assays and highlights the need for such approaches when missense mutations are identified. Genetic primers and the CRFK-VDR⁻ assay system developed in this study may support future diagnoses of feline VDDR-II. Long-term calcium supplementation improved clinical status, though delayed onset of treatment may limit full skeletal recovery.
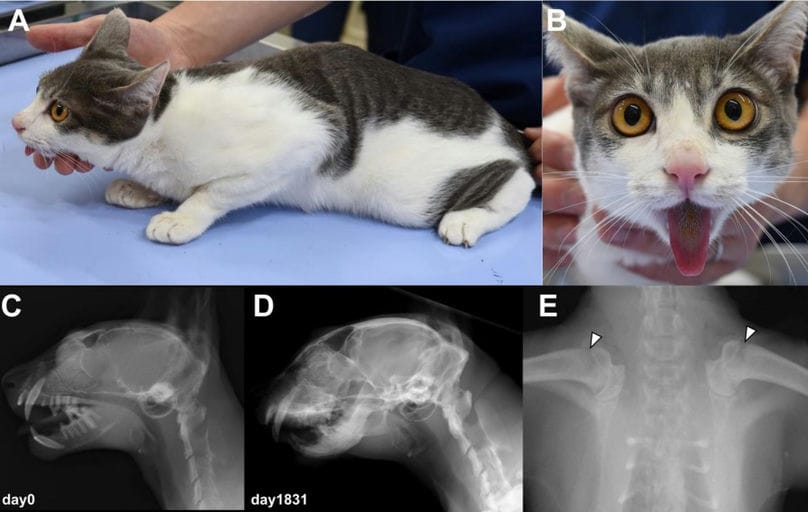
(A, B) The cat at initial presentation. (C, D) Lateral radiograph of the skull before (C) and after
(D) onset of calcium supplementation. Note the increased lucency of the skull and dysplasia of
temporomandibular joint prior to therapy is improved in the images taken during therapy. (E) Ventrodorsal
radiograph of the shoulder at initial presentation. Widened growth plates are indicated by arrowheads.
How did we do? |
Disclaimer: The summary generated in this email was created by an AI large language model. Therefore errors may occur. Reading the article is the best way to understand the scholarly work. The figure presented here remains the property of the publisher or author and subject to the applicable copyright agreement. It is reproduced here as an educational work. If you have any questions or concerns about the work presented here, reply to this email.